
PCCAR-07
for pressure ulcers
Target population:
Patients with pressure ulcers, including those with chronic kidney disease
Pressure ulcers (sores) are foci of necrosis that progress to ulceration of the skin, subcutaneous tissue, and even muscle and bone, which develop due to prolonged pressure and associated tissue hypoxia
These chronic wounds most often develop in areas where bony parts come into contact with the ground
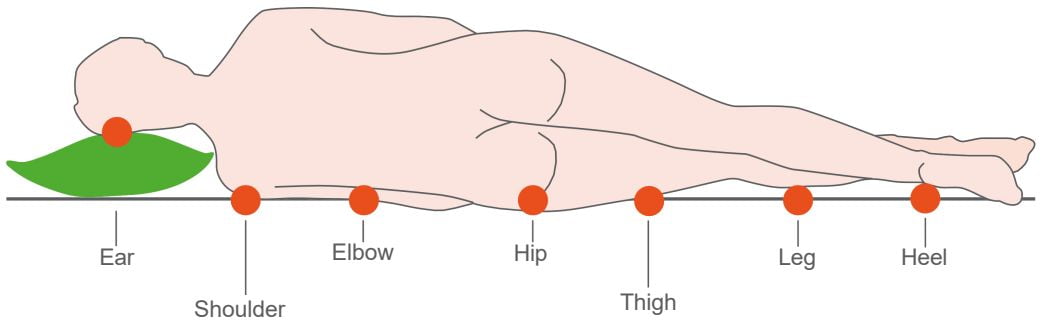
Pressure Ulcers (PUs) – a very serious medical condition
It is frequent: According to a systematic review published in 2019, in 17 European countries and Turkey the average PUs prevalence was estimated as 13.1%
It is very dangerous: PUs double the length of
hospitalization and triple the risk of patient death
Immobility is considered a chief factor in PUs
development, but external pressure relief does not eliminate them
Malnutrition is frequent risk factor of PUs,
and is potentially reversible
Multicomponent dietary supplements with high protein content are recommended for prophylaxis and treatment of PUs
However, approx. one half of patients with PUs suffer from chronic kidney disease and cannot take high protein supplements
PCCAR-07: Our new and smart approach to prevention and treatment of PUs
Wound healing is a complex process obviously dependent on the necessary nutritional substrates
A dietary supplement containing these 4 aminoacids can be given to all patients with PUs, including those who suffer from kidney insufficiency
Carnosine by itself is known to promote healing chronic wounds, and at the same time it has a promising potential for treatment of chronic kidney diseases
In patients with PUs a deficiency of 4 amino acids (alanine, histidine, threonine and tryptophan) has been discovereds
Moreover, one of the deficient amino acids (histidine) can be supplied as a dipeptide carnosine
PCCAR-07: Our new and smart approach to prevention and treatment of PUs

Significant acceleration of PUs healing following oral carnosine (CAR) or its complex with zinc (PLZ) have been shown in a 2013 Japanese paper, but this has been overlooked.
PUSH score is generally accepted method to quantitate pressure ulcers load. Scores measured over time provide an indication of the rate of pressure ulcer healing and allow to evaluate treatment efficacy.
PCCAR-07: Current stage of development and the next steps
IP rights are secured (A nutritional composition intended for patients with pressure ulcers, Polish patent PL235153, EPO procedure is on-going)
Early Clinical Study is expected to be launched in Q2 2023, which will examine the effects of PC-Car07 on the rate of pressure ulcers healing quantified with the PUSH score (primary endpoint) and the levels of the pro-inflammatory cytokines (secondary endpoint)
In the next stage the Late Clinical Study (a randomized, placebo controlled pivotal trial) will be conducted by a Contract Research Organization (CRO) specializing in wound healing
(Cica Biomedical, http://www.cica-biomedical.com/clinical-research.html)


